eSSe Leading the Way
Always on the cutting edge of the latest breakthroughs in plastic surgery are our very own doctors Laura Sudarsky and Tracey H. Stokes who are now in phase two of a clinical trial for a new reconstructive procedure for breast cancer patients. As one of only seven offices in the country offering the trial, eSSe is the only one in Florida to participate, leading the way in innovative reconstructive care.
Dr. Sudarsky had read about trials for the AeroForm Expander and reached out to the company to inquire about participating in the research.
“When I first spoke to them they weren’t taking any new investigators because the FDA had limited the study centers,” said Dr. Sudarsky. “When one of the other centers dropped out, we were given the spot and proceeded enrolling patients.”
Both Drs. Stokes and Sudarsky saw that the AeroForm Expander offered a winning combination for patients in need of breast reconstruction – a reduced amount of healing time as well as a reduction in the number of office visits all while lessening the pain of breast reconstructive surgery. It also allowed the patient to be an active participant in their own care, placing the patient partially in the driver’s seat.
Dr. Sudarsky recognized another benefit, less chance of infection.
“I wanted the opportunity to participate in a trial of a new tissue expander that doesn’t require an injection to fill the expander,” said Dr. Sudarsky. “Many of our patients are on chemo and when they are injected with a needle there is a significant risk of infection. I wanted to try a device that would take that risk out if the expansion.”
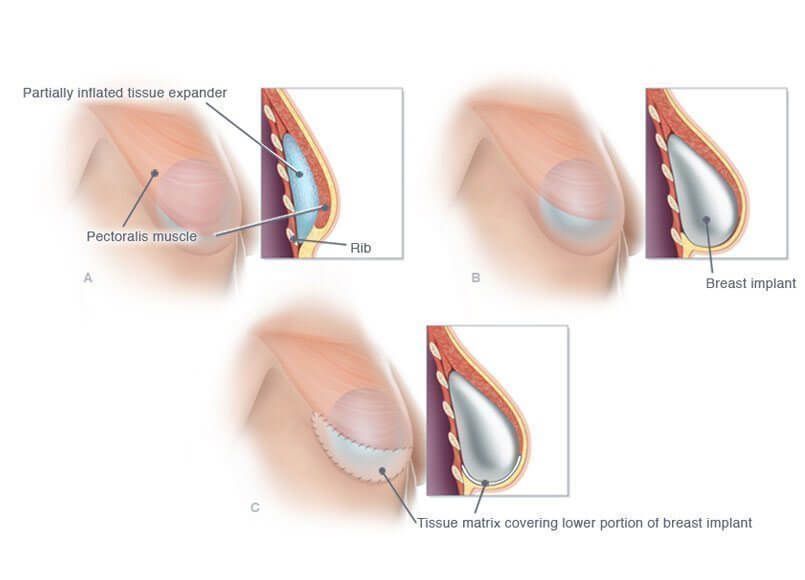
The Expander works like this; the patient is given a wireless remote control to use to gradually self-administer small amounts of compressed carbon-dioxide. The release of this harmless gas slowly stretches the skin and tissue the site of the mastectomy. The slow stretch creates space to place the permanent implant.
Our participating patients are already weighing in, giving it rave reviews for giving them some control over their bodies and their own health care.
“One of the greatest aspects of the AeroForm is that I have been in charge of my own expansion, When you become a cancer patient a great deal of what you control is taken away, therefore it is refreshing to gain access to some control again.”
– Joan
“I was able to expand much faster and control how much I expanded each time. It was nice to have some control over my body.”
-Michele
Using the traditional method, patients would have an expansion device placed after mastectomy and would come into the doctor’s office for weekly injections of saline to expand the tissue and create a pocket for the permanent implant.
The results – less pain, less needles, less visits to the office and quicker healing after the implant is placed.
“The AeroForm allowed me to continue my activities without numerous doctor appointments and the painful injections required for traditional expansion. I was also in charge of my own expansion.”
– Marcy
Our patients, much like our doctors, enjoy being on the forefront of a medical breakthrough.
“After I researched traditional reconstruction with needles, constant trips to doctor, the pain and discomfort, I realized this was an invitation to be a powerful lantern to light a new path for women.”
– Sofia
At eSSe we’re currently screening patients to participate in this trail. A few have already qualified and they’re seeking several more for a total of 10 patients.
“For a patient to qualify she must be a breast cancer patient having a mastectomy and reconstruction,” said Dr. Stokes. “She can’t be a smoker, morbidly obese, or older than 70.”
Currently the AeroForm expander is already in commercial use in Australia where trials have already been done in 2009, 2011 and 2012. The trials were well received showing improved timing over traditional methods.
As for the United States, data from the trial’s first phase is now being reviewed and was presented in Boston at the American Society of Plastic Surgery in October. During the first phase of the trial patients received randomized treatment – some patients were treated using the traditional method while others received the new device. However during phase two testing, all patients will be treated with the AeroForm expander.
The application for FDA approval was submitted this past September, the approval process generally takes about six months.
Both doctors are excited about the possibilities of this new technology and look forward to learning along with their patients all of the benefits the Expander will bring to the reconstruction arena.